RPI seeks to minimize the risks of working in its laboratories for all employees and students. Minimizing risks for pregnant women is especially important due to the sensitivity of the fetus to specific chemicals, biological agents and ionizing radiation. All lab workers should know the hazards of the materials with which they work, and it is important to recognize that an individual’s susceptibility to those hazards may change due to factors such as pregnancy.
Information about specific hazardous materials may be found in Safety Data Sheets (SDSs), labels, literature, from EHS, the lab’s Principal Investigator/Lab Manager, or instructor for the student’s class. All employees, including pregnant women, are encouraged to make use of these sources of information. Safe laboratory procedures minimize exposure for all laboratory employees and, if followed faithfully, they also protect the developing fetus.
The federal Pregnancy Discrimination Act prevents RPI from compelling a woman to disclose that she is pregnant, and it prevents her from being assigned to different tasks simply because she is pregnant. If a woman willingly informs RPI that she is pregnant then additional assessments, precautions or other accommodations can be implemented. Departments and faculty will determine the extent to which a pregnant lab worker or lab student can be excused from lab requirements or what accommodations can be made.

Pregnancy presents a unique risk to chemical researchers due to their occupational exposures to chemical equipment, and physical hazards in research laboratories. Understanding “risk” as a function of hazard, exposure, and vulnerability, is important on ensuring your health and the health of the fetus during pregnancy.
Commonly encountered hazards for pregnant lab workers include chemical hazards (organic solvents, heavy metals, engineered nanomaterials, and endocrine disruptors), radiation hazards (ionizing radiation producing equipment and materials and nonionizing radiation producing equipment), and other hazards related to the lab environment (excessive noise, excessive heat, psychosocial stress, strenuous physical work, and/or abnormal working hours).
Lab relevant doses and routes of exposure in the chemical lab environment along with literature and governmental recommendations or resources for exposure mitigation are critically assessed. The specific windows of vulnerability based on stage of pregnancy are described for each hazard, if available.
A safe laboratory setting should minimize most risks; however, no workplace can eliminate all risks, and pregnancy should be factored into a personal risk assessment. Because research is highly specialized, the most important first steps you can take are to realistically assess your potential laboratory risks and to consult with a medical professional to discuss your health concerns.
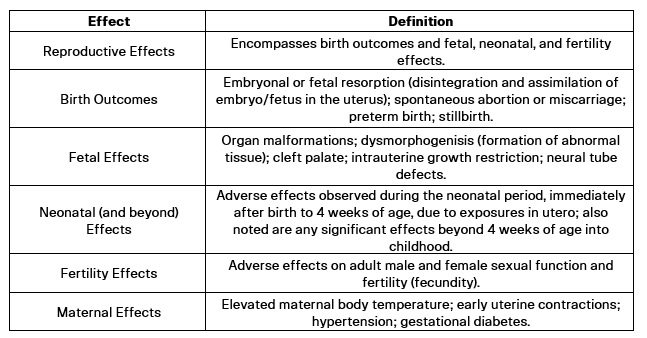
When examining hazards, numerous adverse effects related to reproduction and pregnancy emerge, which can be categorized into several adverse effects with common outcomes.
EH&S can assist you in identifying your research-related risks and discuss potential mitigation strategies. This knowledge can then be shared with your medical physician to better evaluate your potential health concerns.
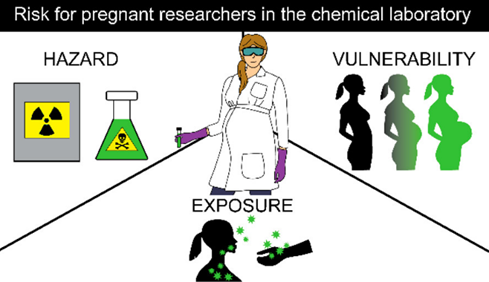
The concern with chemicals and chemical processes toward pregnancy outcomes arises from effects on both maternal and fetal health. Risk associated with chemical laboratory work for pregnant researchers depends on the level of hazard, exposure, and vulnerability, where each exists on a scale that contributes to overall risk. Main routes of exposure are dermal, oral, and inhalation exposure, along with ambient exposure to hazardous environments such as radiation or sound. A pregnant researcher and their developing fetus can be more or less vulnerable to certain hazards and exposures based on the progression of their pregnancy.
Organic solvents, heavy metals, and engineered nanomaterials pose an elevated risk to pregnant women and their fetuses due to their volatility, chemical properties, and toxicity. Knowing the risks involved with these categories of chemicals and how to mitigate those risks is essential in continuing work with these chemicals.
Common organic solvents encountered in a chemical laboratory with Global Harmonization System Classification for reproductive toxicity and adverse reproductive effects on humans and common heavy metals encountered in a chemical laboratory and their reproductive effects are listed by the American Chemical Society.
Some biological agents pose increased risks for a developing fetus relative to a healthy adult. Working with certain biological agents can increase the chances of having a miscarriage or a child with birth defects. Some pathogens can also be amplified in pregnant women, and these pathogens should be handled with increased precaution.
At a minimum, standard precautions must be taken to protect against bacteriological hazards. These include wearing appropriate PPE, washing hands, performing routine cleanup of the lab, and handling biomedical waste appropriately. When working in a lab with biological hazards, continue to follow the laboratory safety procedures outlined in the RPI Biosafety Manual.
Exposure of a developing fetus to ionizing radiation is assumed to carry a risk of causing certain adverse health effects. The occurrence and severity of these health effects depend on radiation type, total dose, stage of pregnancy, and the time period over which the exposure was received. The first trimester is known to be the most radiosensitive time for a fetus, thus, it is beneficial, but not required, to meet with the Radiation Safety Officer (RSO) as soon as possible to review safety practices and monitoring options. If a pregnant radiation worker decides to declare her pregnancy, she will meet with the RSO to review radiation safety procedures, the risk to the fetus, and the RPI Radiation Safety Program.
The Nuclear Regulatory Commission (NRC), 10CFR § 20.1208, places different radiation dose limits on declared pregnant workers than on other adult workers. Specifically, a declared pregnant worker who chooses to continue working as an occupational worker has a dose limit for the fetus from conception to birth (entire gestation period) of 500 mrem.
A pregnant researcher who does not declare that she is pregnant is protected under the regulations for adult radiation workers only and the dose limit for the fetus does not apply.
Pregnant lab workers can:
- Request a hazard assessment with EHS to understand those potential exposures and protective measures that should be utilized
- Request from the Department, an altered assignment within the lab either through a change in lab duties, a reduced time-frame within the lab or a change in location*
- Request a delay in entry into the academic program requiring the laboratory work*
- Continue to work in a laboratory by utilizing the regular safety precautions that have been developed for the laboratory.
*Please note that requests for altered schedules might not be able to be honored.*
American Chemical Society - What to Expect When Expecting in Lab: A review of Unique Risks and Resources for Pregnant Researchers in the Chemical Laboratory
